Previous Year GATE Solved Questions on Work, Heat and Power
Question 1. A cyclic heat engine does 50 KJ of work per cycle. If the efficiency of the heat engine is 75 %, the heat rejected per cycle is
(A) 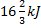
(B) 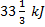
(C) 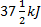
(D) 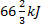
GATE-ME-2001
Hint 1. (Ans A)
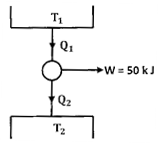
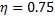
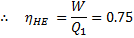
Or, Net heat input ,
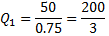
Also,
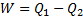
Or
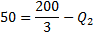
Therefore Net rejected heat,

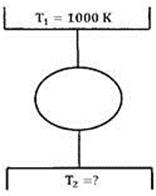
Question 2. A Carnot cycle is having an efficiency of 0.75. If the temperature of the high temperature reservoir is 727°C, what is the temperature of the low temperature reservoir?
(A) 23 °C
(B) -23°C
(C) 0°C
(D) 250°C
GATE-ME-2002
Hint 2. (Ans B)
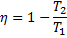
Or

Therefore

Data for Q.3-4 are given below. Solve the problem and choose correct answer.
Nitrogen gas (molecular weight 28) is enclosed in a cylinder by a piston, at the initial condition of 2 bar, 298 K and 1  . In a particular process, the gas slowly expands under isothermal condition, until the volume becomes 2
. In a particular process, the gas slowly expands under isothermal condition, until the volume becomes 2  .Heat exchange occurs with the atmosphere at 298 K during this process.
.Heat exchange occurs with the atmosphere at 298 K during this process.
Question 3. The work interaction for the nitrogen gas is
(A) 200 KJ
(B) 138.6 KJ
(C) 2 KJ
(D) -200 KJ
GATE-ME-2003
Hint 3. (Ans B)
Work interaction,

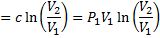
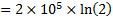
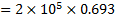

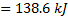
Question 4. The entropy change for universe during the process in KJ/K is
(A) 0.4652
(B) 0.0067
(C) 0
(D) -0.06711
GATE-ME-2003
Hint 4. (Ans A)

For isothermal process,

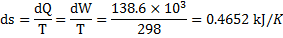
Question 5. A gas contained in a cylinder is compressed, the work required for compression being 5000 KJ. During the process, heat interaction of 2000 KJ causes the surroundings to be heated. The change in internal energy of the gas during the process is
(A) -7000 KJ
(B) -3000KJ
(C) +3000 KJ
(D) +7000 KJ
GATE-ME-2004
Hint 5. (Ans C)


Question 6. Match items from groups I,II, III, IV and V.
| Group I | Group II | Group III | Group IV | Group V |
| When added to the system, is | Differential | Function | Phenomenon | |
| E Heat | G positive | I Exact | K Path | M Transient |
| F work | H Negative | J Inexact | L Point | N Boundary |
(A) F-G-J-K-M
E-G-I-K-N
(B) E-G-I-K-M
F-H-I-K-N
(C) F-H-J-L-N
E-H-I-L-M
(D) E-G-J-K-N
F-H-J-K-M
GATE-ME-2006
Hint 6. (Ans D)
|
When added to the system |
Differential |
Function |
Phenomenon |
|
|
Heat |
Positive |
Inexact |
Path |
Boundary |
|
Work |
Negative |
Inexact |
Path |
Transient |
Common Data Question: 7 & 8
A thermodynamic cycle with an ideal gas as working fluid is shown below.
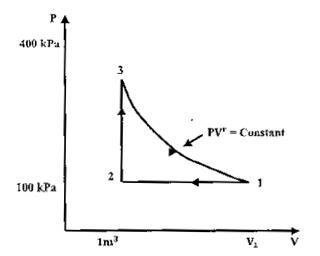
Question 7. The above cycle is represented on T-S plane by
(A) 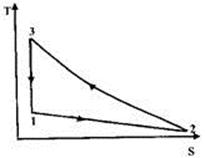
(B) 
(C) 
(D) 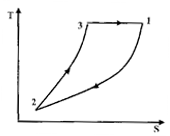
GATE-ME-2007
Hint 7. (Ans C)
Since 3-1 is adiabatic process, it will be represented by a straight line on T-s plane. 1-2 is isobaric process, so with decrease of volume, temperature will also decrease.
Question 8. If the specific heats of the working fluid are constant and the value of specific heat ratio 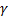 1.4, the thermal efficiency (%) of the cycle is
1.4, the thermal efficiency (%) of the cycle is
(A) 21
(B) 40.9
(C) 42.6
(D) 59.7
GATE-ME-2007
Hint 8. (Ans A)
Question 9. A heat transformer is a device that transfers a part of the heat, supplied to it at an intermediate temperature, to a high temperature reservoir while rejecting the remaining part to a low temperature heat sink. In such a heat transformer, 100 KJ of heat is supplied at 350 K. the maximum amount of heat in KJ that can be transferred to 400 K, when the rest is rejected to a heat sink at 300 K is
(A) 12.50
(B) 14.29
(C) 33.33
(D) 57.14
GATE-ME-2007
Hint 9. (Ans D)
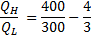

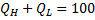
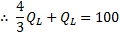
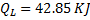

Common data for Questions 10,11 and 12:
In the figure shown, the system is a pure substance kept in a piston cylinder arrangement. The system is initially a two phase mixture containing 1 kg of liquid and 0.3 kg of vapour at a pressure of 100 kPa. Initially, the piston rests on a set of stops , as shown in the figure. A pressure of 200 kPa is required to exactly balance the weight of the piston and the outside atmospheric pressure. Heat transfer takes place into the system until its volume increases by 50 %. Heat transfer to the system takes place in such a manner that the piston, when allowed to move, does so in a very slow (quasi-static/quasi-equilibrium) process. The thermal reservoir from which heat is transferred to the system has a temperature of 400°C. Average temperature of the system boundary can be taken as 175°C. The heat transferred to the system is 1 KJ, during which its entropy increase by 10 J/K.
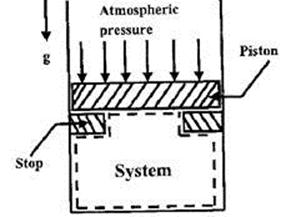
Specific volume of liquid ( ) and vapour (
) and vapour (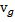 ) phases, as well as the value of the saturation temperatures, are given below.
) phases, as well as the value of the saturation temperatures, are given below.
|
Pressure (kPa) |
Saturation Temperature
|
|
|
|
100 |
100 |
0.001 |
0.1 |
|
200 |
200 |
0.0015 |
0.002 |
Question 10 .At the end of the process, which one of the following situations will be true?
(A) Superheated vapour will be left in the system
(B) No vapour left in the system
(C) A liquid +vapour mixture will be left in the system
(D) The mixture will exist at a dry saturate vapour state
GATE-ME-2008
Hint 10. (Ans A)
Given data:
Mass of liquid  =1 kg
=1 kg
Mass of vapour 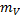 = 0.03 kg
= 0.03 kg
Pressure of two phase mixture = 100 kPa
Dryness fraction of the steam
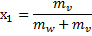
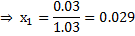
Dryness fraction of steam at 200 kPa (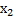 )
)

Question 11. The work done by the system during the process is
(A) 0.1 KJ
(B) 0.2 KJ
(C) 0.3 KJ
(D) 0.4 KJ
GATE-ME-2008
Hint 11. (Ans D)
W.D by the system (W)=W.D. in constant volume process +W.D in constant pressure process
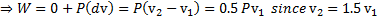

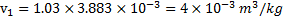

Question 12. The net entropy generation (considering the system and the thermal reservoir together) during the process is closest to
(A) 7.5 J/K
(B) 7.7 J/K
(C) 8.5 J/K
(D) 10 J/K
GATE-ME-2008
Hint 12. (Ans C)
Net entropy
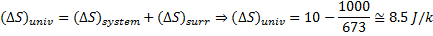
Question 13. A gas expands in a frictionless piston-cylinder arrangement. The expansion process is very slow, and is resisted by an ambient pressure of 100 kPa. During the expansion process, the pressure of the system (gas) remains constant at 300 kPa. The change in volume of the gas is 0.01  . That maximum amount of work that could be utilized from the above process is
. That maximum amount of work that could be utilized from the above process is
(A) 0 KJ
(B) 1 KJ
(C) 2 KJ
(D) 3 KJ
GATE-ME-2008
Hint 13. Maximum amount of work
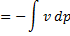
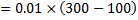
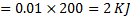
Question 14. Two moles of oxygen are mixed adiabatically with another 2 moles of oxygen in mixing chamber, so that the final total pressure and temperature of the mixture become same as those of the individual constituents at their initial states. The universal gas constant is given as R. The change in entropy due to mixing, per mole of oxygen, is given by
(A) 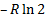
(B) 0
(C) 
(D) 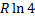
GATE-ME-2008
Hint 14. (Ans B)
Since 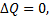 therefore
therefore 
Question 15. A frictionless piston cylinder device contains a gas initially at 0.8 MPa and 0.015 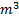 . It expands quasi-statically at constant temperature to a final volume of 0.030
. It expands quasi-statically at constant temperature to a final volume of 0.030 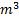 . The work output (in KJ) during this process will be
. The work output (in KJ) during this process will be
(A) 8.32
(B) 12.00
(C) 554.67
(D) 8320.00
GATE-ME-2009
Hint 15. (Ans A)
In isothermal process
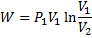
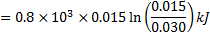

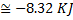
Alternately


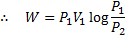


Question 16. If a closed system is undergoing an irreversible process, the entropy of the system
(A) Must increase
(B) Always remains constant
(C) Must decrease
(D) Can increase, decrease or remain constant
GATE-ME-2009
Hint 16. (Ans D)
If a closed system is undergoing an irreversible process, the entropy of the system can increase, decrease or remain constant.
Question 17. A mono-atomic ideal gas (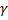 =1.67, molecular weight=40) is compressed adiabatically from 0.1 MPa, 300 K to 0.2 MPa. The universal gas constant is 8.314
=1.67, molecular weight=40) is compressed adiabatically from 0.1 MPa, 300 K to 0.2 MPa. The universal gas constant is 8.314 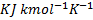 . The work of compression of the gas (in
. The work of compression of the gas (in 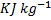 ) is
) is
(A) 29.7
(B) 19.9
(C) 13.3
(D) 0
GATE-ME-2010
Hint 17. (Ans A)

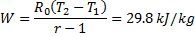
Question 18. One kilogram of water at room temperature is bought into contact with a high temperature thermal reservoir. The entropy change of the universe is
(A) Equal to entropy change of the reservoir
(B) Equal to entropy change of the water
(C) Equal to zero
(D) Always positive
GATE-ME-20010
Hint 18. (Ans D)
The entropy change of the universe is always positive.
Question 19. An ideal gas of mass m and temperature 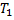 undergoes a reversible isothermal process from an initial pressure
undergoes a reversible isothermal process from an initial pressure 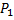 to final pressure
to final pressure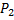 . The heat loss during the process is Q. the entropy change
. The heat loss during the process is Q. the entropy change  of the gas is
of the gas is
(A) 
(B) 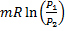
(C) 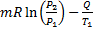
(D) Zero
GATE-ME-2012
Hint 19. (Ans C)
Answer keys
1. (A) 2. (B) 3. (B) 4. (B) 5. (C) 6. (D) 7. (C) 8. (A)
9. (D) 10. (A) 11. (D) 12. (C) 13. (C) 14. (B) 15. (A) 16. (D)
17. (A) 18. (D) 19. (C)
 (
(

One Response to “Previous Years GATE MCQ’s on Work, Heat and Power”
Anyewi George
Mechanical