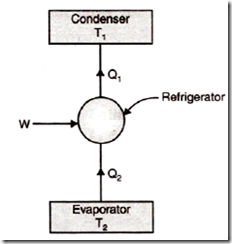
Vapor Compression Refrigeration
Introduction: Vapour compression refrigeration is the most important and practical form of refrigeration system for domestic and commercial utility.
Refrigerant: The working fluids (refrigerants) used are NH3, CO2, Freons etc., and these fluids have the characteristic ability to change alternately between the vapour and liquid phases without leaving the plant.
Working Method: The alternate condensation and evaporation takes place at temperature and pressure close to atmospheric conditions, during evaporation, the liquid refrigerant absorbs its latent heat from the space/product to be cooled and changes from the liquid to gaseous state. The vapour is next compressed (raised in temperature and pressure) and subsequently condensed. While condensing, the vapour liquefies by rejecting heat to an external system (typically available cooling water or cooling air). As such, this refrigeration system may be likened to a latent heat pump that pumps heat from the space being refrigerated to either water or air in the condenser. The system is called vapour compression as it is the compression of refrigerant that permits pumping of heat.
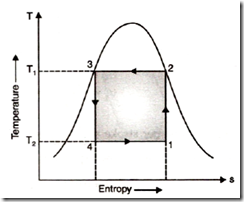
Working Process in a vapour compression Refrigeration:
Process 1-2: The refrigerant as a mixture of liquid and vapour corresponding to state point 1 enters the compressor where isentropic compression takes place. The compression process increases the temperature of refrigerant from lower limit T2 to the upper limit Tl. Work is supplied to the system and after compression, the vapour is wet or saturated but not superheated.
Process 2-3: The refrigerant in the form of vapour enters the condenser at state 2 and heat is rejected at constant pressure and temperature. At exit from the condenser, the refrigerant becomes saturated liquid at state point 3.
Process 3-4: The refrigerant at state point 3 enters the expansion cylinder expands isentropic ally and its temperature drops to lower temperature T2 at the end of the expansion process. Work is obtained during the expansion process.
Process 4-1: The liquid refrigerant at point 4 enters the evaporator and extracts heat at constant pressure and temperature from the space or substance being cooled and thus produces refrigerating effect.
The cycle consists of two isothermals and two adiabatic and obviously is a reversible cycle. From the temperature-entropy plot,
Heat absorbed (refrigerating effect) during evaporation process (4 – 1)
Q2 = T2 x ds
Heat rejected during condensation process (2 – 3)
Ql = Tl x ds
Work input W = heat rejected – heat absorbed = (T1 – T2) ds
2 Responses to “Vapor Compression Refrigeration”
Albert
Please correct above mistake.
(COP) Ref = T2 / (T1-T2)
THANK YOU !
pritpal singh
Good