Steam :
When we provide continuous heat to water then at 100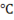 temperature and 1 atm pressure, it boils and changes its phase from liquid to vapour. This vapour is known as steam.
temperature and 1 atm pressure, it boils and changes its phase from liquid to vapour. This vapour is known as steam.
Steam contains more energy as it has both sensible heat and latent heat of vaporization. Steam has been a popular mode of conveying energy since the industrial revolution. Steam isused for generating power and also used in process industries such as sugar, paper, fertilizer, refineries, petrochemicals, chemical, food, synthetic fibre and textiles The following characteristics of steam make it so popular and useful to the industry:
• Highest specific heat and latent heat
• Highest heat transfer coefficient
• Easy to control and distribute
• Cheap and inert
Types : Wet steam, Dry steam, Superheated steam
Wet Steam: When steam contains water particles then it is known as Wet steam
Dry Steam: When wet steam is further heated then all water particles get converted into vapour and resulted steam is called dry steam.
Superheated Steam: When dry saturated steam is heated to higher temperatures then steam obtained is in superheated state. This steam is mostly used in Power generation.
Use :
(1) Power generation
(2) Heat engines (i.e. steam engines train)
Formation of steam at constant pressure:
Before going into formation of steam let we understand why steam is being formed/produced at constant pressure.
The boiling temperature is 100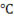 at one particular pressure that is 1 atm. Now if we change the pressure then the boiling temperature also changes. Since both are directly proportional then if we decrease pressure, boiling temperature decreases and vice versa.
at one particular pressure that is 1 atm. Now if we change the pressure then the boiling temperature also changes. Since both are directly proportional then if we decrease pressure, boiling temperature decreases and vice versa.
T-h diagram of formation of steam at constant pressure :
After having understood the temperature and pressure fundamentals, let we discuss how we are going to produce steam from 1 kg piece of ice.
Suppose the initial temperature of ice is a solid phase of water is -10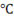 .Now, keeping pressure constant which is again assumed as 1 atm, we provide continuous heat to the ice.
.Now, keeping pressure constant which is again assumed as 1 atm, we provide continuous heat to the ice.
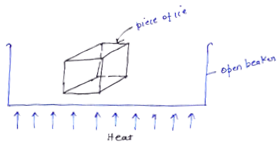
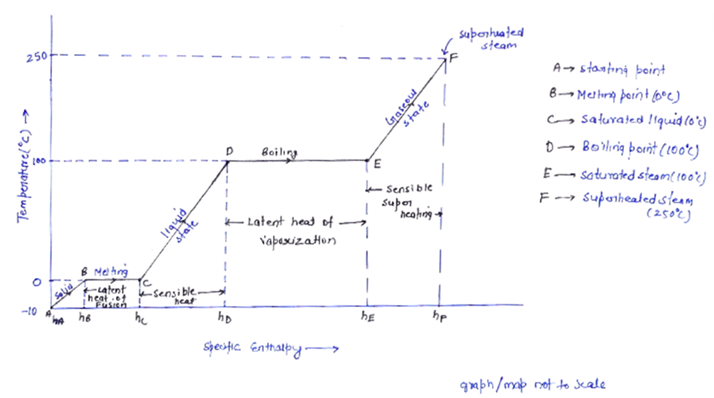
Let us understand what is happening in the above graph.
The graph is plotted between T and h. Let’s start with point A at which ice is at temperature -10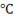 . Now, as we provide heat to the piece of ice, its temperature as well as enthalpy increases simultaneously upto point ‘B’.
. Now, as we provide heat to the piece of ice, its temperature as well as enthalpy increases simultaneously upto point ‘B’.
Point B is at 0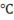 at which the phase transformation of solid ice into liquid takes place. This point is called melting point and temperature will remain constant until the whole transformation process is completed i.e all ice will convert in liquid at 0
at which the phase transformation of solid ice into liquid takes place. This point is called melting point and temperature will remain constant until the whole transformation process is completed i.e all ice will convert in liquid at 0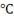 . This is so because of latent heat of fusion. All the heat we are providing is actually increasing the energy of the molecules of the water not its temperature, that’s why enthalpy increases but the temperature remains constant upto point C. Heat provided from point ‘B’ to point ‘C’ is hidden and cannot be measured using thermometer.
. This is so because of latent heat of fusion. All the heat we are providing is actually increasing the energy of the molecules of the water not its temperature, that’s why enthalpy increases but the temperature remains constant upto point C. Heat provided from point ‘B’ to point ‘C’ is hidden and cannot be measured using thermometer.
Since we are providing continuous heat the temperature and the enthalpy both increases (sensible heating). At ‘D’ which is boiling point of water. The next phase transformation i.e. liquid to vapour (steam) takes place. Since, the temperature again remains constant and enthalpy keep increasing, the heat absorbed during this is latent heat of vaporization. This process goes upto ‘E’. At ‘E’ we have converted all liquid into steam. If we keep providing heat then ultimately at ‘F’ superheated steam is obtained.
Thermodynamic properties of steam :
It is necessary to understand the properties of steam so that we can make proper use of it. Equipment size can be decided, pipe systems can be perfectly designed. It also allows us to make informed decisions affecting the energy usage of the system.
The properties are:
(1) Quality of steam (Dryness fraction)
Dryness fraction in simple words denotes the mass of dry steam in given steam. Or how much steam is dry or in other words how much water vapour is present in steam. It is denoted by ‘x’.
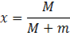
Where M=mass of the dry steam
m=mass of water vapour
The use of dryness fraction allow us to know both the mass of dry steam and mass of water vapour.
Now, see
If 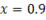 that means dry steam is 0.9 kg and water vapour is 0.1 kg in 1 kg of given steam.
that means dry steam is 0.9 kg and water vapour is 0.1 kg in 1 kg of given steam.
Obviously for dry steam, 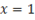
Quality is represented in percentage but meaning is same as ‘x’.
If quality of steam is 80%, then it has 80% of dry steam and 20 % water vapour by mass.
(2) Specific volume :
Gases (steam is a gas) occupy less space under higher pressure than under lower pressure. This means 1 kilogram of steam occupies different volumes, depending upon its pressure. The term specific volume refers to the volume that one kg of steam occupies at a given pressure and temperature.
Unit is 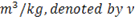
(3) Enthalpy
The total heat content of a substance is called enthalpy. Actually it has much broad definition in thermodynamics but for 1st year BME students this definition works just fine. So, total heat content by steam is termed as its enthalpy. It is denoted by ‘H’. SI unit is KJ
‘h‘ is generally used term which represents specific enthalpy, unit for which is KJ/Kg.
In steam tables you will see enthalpy written as 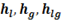
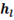 is the enthalpy of liquid that is water at boiling point that’s why subscript ‘l’ is used, point ‘D’ in h-T diagram. Similarly
is the enthalpy of liquid that is water at boiling point that’s why subscript ‘l’ is used, point ‘D’ in h-T diagram. Similarly 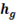 is enthalpy of dry saturated steam, point ‘E’ in h-T diagram and
is enthalpy of dry saturated steam, point ‘E’ in h-T diagram and 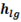 is the latent heat, Process D to E in h-T diagram.
is the latent heat, Process D to E in h-T diagram.
Use of steam tables
Till now we had studied that it is very important to study and understand the properties of steam for proper utilization. Steam tables enable us to know these properties directly i.e. without doing any calculation or other thing. These properties can be found out at a given pressure or temperature.
Before obtaining these values let us understand few more things
Saturated steam :
Saturated steam is pure steam at the temperature that corresponds to the boiling temperature of water at the existing pressure.
For example : Saturated steam is obtained at point ‘E’. where all the liquid water has converted into steam.
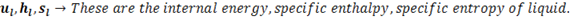
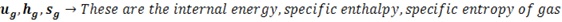
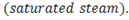
Coming back to steam table use, we can use either temperature tables or pressure tables.
If we have to find properties of steam at given temperature, use temperature scale and vice-versa.
For super-heated steam, different table is given. Keep units in mind while using steam tables and use scale for current measurement
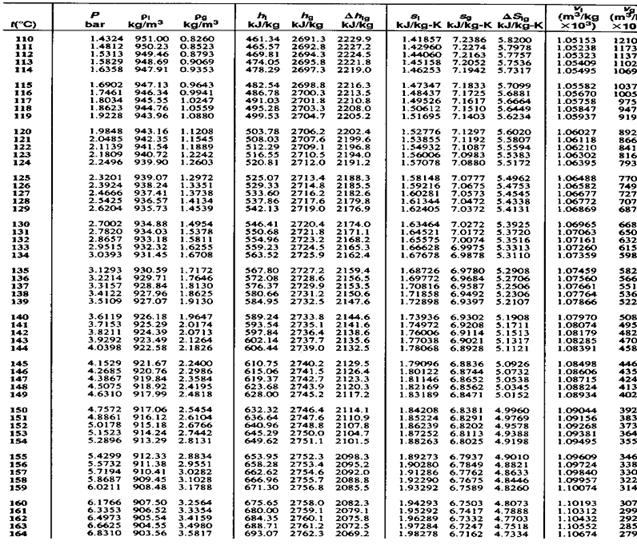
Example on steam table usage
Suppose you have to find all properties of steam at 150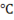 . Then first of all use temperature scale.
. Then first of all use temperature scale.
Find out in the temperature where 150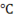 is. Then mark it and put a scale across it covering the entire row. This entire row will give you all the properties corresponding to this temperature.
is. Then mark it and put a scale across it covering the entire row. This entire row will give you all the properties corresponding to this temperature.
Fig. in this way, we can get all the properties,  .
.
So from above table at 150 values of properties of steam are
values of properties of steam are

Throttling Calorimeter
Before explaining how the throttling calorimeter works we first explain the process of throttling in some detail.
Throttling
A throttling process is one in which the fluid is made to flow through a restriction, e.g. a partially opened valve or an orifice plate, causing a considerable loss in the pressure of the fluid. Diagrams for these two examples are shown below.
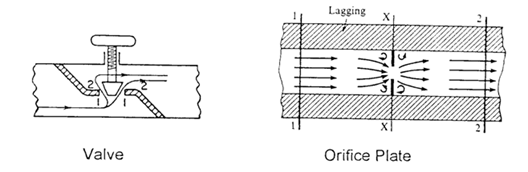
Calculations for the process are based on a formulation derived from the steady flow energy equation (SFEE),
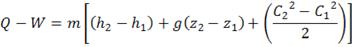
In this process there is no change in elevation so z2=z1, so the potential energy term is zero. The velocity C2 is similar to C1, so the change in the kinetic energy is neglected. If the system is suitably insulated then there is no heat transfer so Q= 0. Finally, the system does no external work, i.e. W =0. So we are left with simply
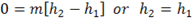
In other words, during a throttling process the enthalpy remains constant.
A diagram of the Throttling Calorimeter is shown below. The throttling calorimeter makes use of the fact that the enthalpy before throttling is the same as that after throttling, in order to determine the dryness fraction, x, of the steam passing through the calorimeter. The wet steam passes through a small orifice where it must undergo a sufficiently large pressure drop to become superheated. In other words it is allowed to expand sufficiently so that all of the liquid part becomes vapour.
The dryness fraction is determined as follows. If we know the pressure within the main steam supply pipe of the calorimeter then we can find the dry and wet saturation values of the enthalpy from the steam tables, hg1 and hf1, respectively. If we know the temperature and the pressure in the expansion chamber then we can tell if all of the steam has changed to vapour. For at a certain temperature, the saturated vapour will have a certain pressure (look at the values in the left hand column of the saturation tables). So the enthalpy of this dry steam will be hg2. We can then find the dryness fraction from
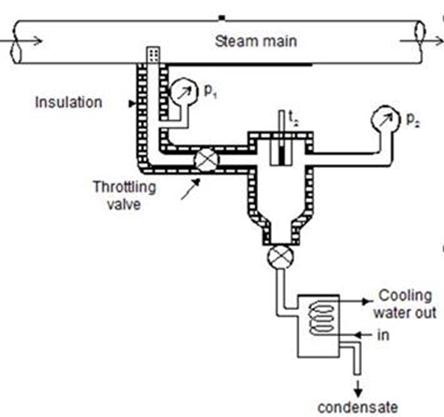
Throttling Calorimeter
The limitation of the throttling calorimeter is that it relies on the steam being totally vapour in the expansion chamber. In practice it may not be and one solution is a combination “Separation and Throttling Calorimeter”. This also solves the problem of some of the water droplets going out of the steam pipe in the Separating Calorimeter.
For dryness fraction, we have to do calculations but that’s beyond the syllabus of BME. You will do it in thermodynamics in 3rdsem.
6 Responses to “Propertis of Steam”
ritz itz sharma
Thank you for helping me p
Rejith
Thank you very much…it’s really useful
Shalini
Tq it’s is very useful
Shalini
Tq it’s very useful
Shatrughan yadav
I m from mechanical engineering and thanks alot to u sir to provide this article .It is very helpful to me and may be to all other students
Nura bb
Thank you it’s very interested