GATE Solved Questions on Irreversibility and Availability
Question 1. A gas having a negative Joule-Thompson coefficient (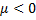 ), when throttled, will
), when throttled, will
(A) Become cooler
(B) Become warmer
(C) Remain at the same temperature
(D) Either be cooler or warmer depending on the type of gas
GATE-ME-2001
Hint 1. (Ans B)
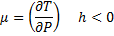
Since in throttling is decrease in pressure,
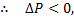
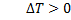
Question 2. A positive value of Joule-Thompson coefficient of a fluid means
(A) Temperature drops during throttling
(B) Temperature remains constant during throttling
(C) Temperature rises during throttling
(D) None of these
GATE-ME-2002
Hint 2. (Ans A)
Question 3. Considering the relationship Tds=dU+pdV between the entropy (S), internal energy (U), pressure (p), temperature (T) and volume (V), which of the statement is correct.
(A) It is applicable only for a reversible process
(B) For an irreversible process TdS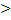 dU+pdV
dU+pdV
(C) It is valid only for an ideal gas
(D) It is equivalent to 1st law, for a reversible process
GATE-ME-2003
Hint 3. (Ans D)
Question 4. A steel billet of 2000 kg mass is to be cooled from 1250 K to 450 K. The heat released during this process is to be used as a source of energy. The ambient temperature is 303 K and specific heat of steel is 0.5 kJ/kg K. The available energy of the billet is
(A) 490.44 MJ
(B) 30.95 MJ
(C) 10.35 MJ
(D) 0.10 MJ
GATE-ME-2004
Hint 4. (Ans A)
Given : 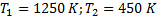
Specific heat, c=0.5 kJ/kg K,
Ambient temperature, 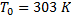
Mass of billet, m=2000 kg
Available energy
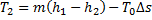
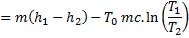
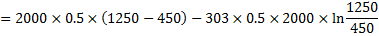
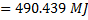
Question 5. Nitrogen at an initial state of 10 bar, 1  and 300 K is expanded isothermally to a final volume of 2
and 300 K is expanded isothermally to a final volume of 2 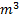 the P-v-T relation is
the P-v-T relation is 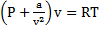 , where
, where 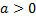 . The final pressure
. The final pressure
(A) Will be slightly less than 5 bar
(B) Will be slightly more than 5 bar
(C) Will be exactly 5 bar
(D) Cannot be ascertained in the absence of the value of a.
GATE-ME-2005
Hint 5. (Ans B)
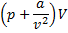
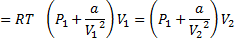
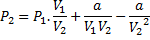
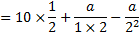
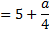
Question 6. Which combination of the following statement is correct?
P : A gas cools upon expansion only when its Joule-Thompson co-efficient is positive in the temperature range of expansion.
Q : For a system undergoing a process, its entropy remains constant only when the process is reversible.
R : The work done by a closed system in an adiabatic process is a point function.
S : A liquid expands upon freezing when the slope of its fusion curve on pressure-temperature diagram is negative..
(A) R and S
(B) P and Q
(C) Q, R and S
(D) P, Q and R
GATE-ME-2007
Hint 6. (Ans B
Question 7. Consider the following two processes;
I. A heat source at 1200 K loses 2500kJ of heat to a sink at 800 K
II. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 K
which of the following statement is true?
(A) Process I is more irreversible than process II
(B) Process II is more irreversible than process I
(C) Irreversibility associated in both the processes are equal
(D) Both the process are reversible
GATE-ME-2010
Hint 7. (Ans B)
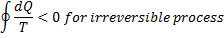
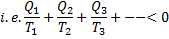
Process I
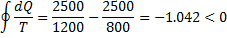
Process II
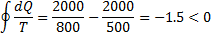
Therefore, process II is more irreversible than process I.
Answer keys
1. (B), 2. (A), 3. (D), 4. (A) 5. (B) 6. (B), 7. (B)
2 Responses to “Previous years GATE MCQ’s on Irreversibility and Availability”
Amal
Its great:)!!!
admin
Thanks