Properties of Pure Substances
Part 1
State postulate:
Let us consider a system in a particular state. A change of state can take place due to energy transfer across the boundary of the system or by removal of internal constraints, if any.
In the later case, a change of state occurs but, the total internal energy remains constant. In a general case, the change of state may take place due to both the above. In such a case, changes may take place in the system properties including internal energy.
In fact, the second type of change of state (by removal of internal combustion) can usually be brought about by energy transfer as heat and work. The state of any system may be changed through energy transfer by reversible or irreversible modes of work.
For any reversible work mode of energy transfer, there is an associated system property that changes. A change of state will therefore involve, at least, as many property changes as the number of possible reversible work modes.
For irreversible work mode or heat transfer, there is no associated property that must necessarily change. However, during any type of energy transfer, a change in internal energy takes place, as per conservation of energy (First Law of Thermodynamics).
Also, when the system reaches a new equilibrium state after the energy transfer, it will be impossible to say whether the energy transfer took place as work or heat. This is because the same final condition may be brought about by energy transfer either as heat or work. Therefore, for irreversible energy transfer, only one extra property change need be specified, in addition to those associated with the reversible work modes, to fix the final state of the system.
We may express the State Postulate thus:
The state of the system is fixed completely by specifying any (n+1) independent properties, where n is the number of reversible work modes associated with the system.
The reversible work modes considered should be relevant to the system under consideration. For example, pdV work is irrelevant to an incompressible substance. Hence, pressure is not a relevant property in such a case.
Thermodynamic properties of pure substances in solid, liquid and vapour phases, p-v-t behaviour of single compressible substances:
A simple substance involves only one possible work mode. A non-ionised gas with no magnetic dipole moment in such a substance. The only possible work mode would be pdV work.
A pure substance is one which is homogeneous and has the same chemical composition in all the phases. Water is a pure substance. A mixture of gases, like air, behaves like a pure substance unless cooled to such a temperature at which one or more of the components (but not all) condense into liquid, thereby disturbing the chemical composition of the gas/vapour phase.
The state of a simple pure substance in thermodynamic equilibrium can be fixed by specifying two properties. It means that we need to know only two, out of pressure, temperature and specific volume, to fix the state of a pure substance.
A pure substance can exist in any of the 3 phases (solid, liquid or vapour) or a combination of these. For instance, the most common substance, water exists in the following phases:
a. Solid phase – ice
b. Liquid phase – Water
c. Vapour phase – Steam
d. An equilibrium mixture of liquid and vapour phases
e. An equilibrium mixture of solid and liquid phases
f. An equilibrium mixture of solid and vapour phases
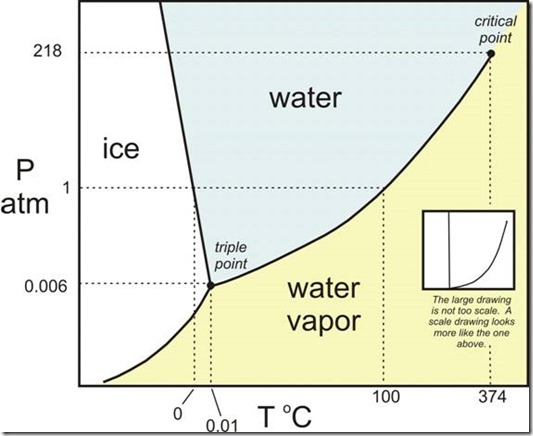
The following figure (figure 1) shows the various phases of water on a pressure-temperature diagram
Figure 1: Pressure-Temperature diagram for a substance such as water
The change of state from solid to liquid is known as fusion and the change from liquid to vapour is called vaporisation.
Sublimation is the name given to the change of state from solid to vapour. In each of these processes, energy must be added to the substance to effect the change of phase. The temperature at which the change from one state to another depends on the pressure exerted on the substance.
The pressure and temperature at which all three phases exist in equilibrium is called the Triple Point.
The line at ‘1’ in figure 1 is a constant pressure line at atmospheric pressure. Water can exist in any of these phases at atmospheric pressure as shown, depending on the temperature.
The Critical point is he state where pure vapour phase has identical properties with a pure liquid phase at the same pressure and temperature.
The three lines shown in figure 1 (fusion, vaporisation and sublimation) are the equilibrium lines and designate the saturation regions.
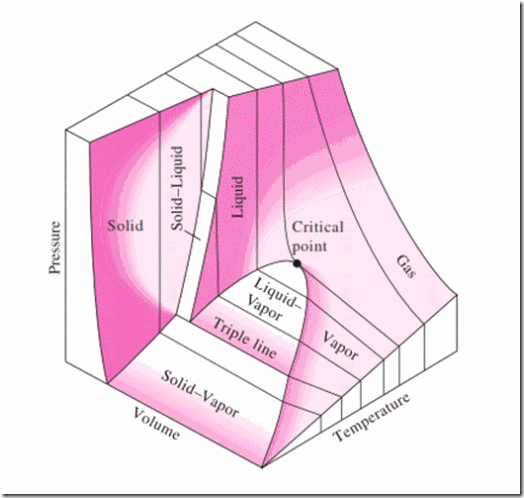
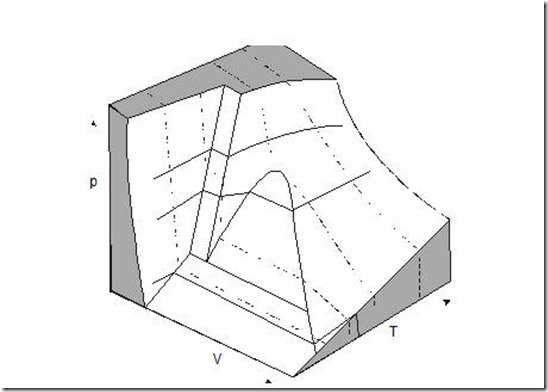
We shall now consider a three dimensional diagram on p, v and T coordinate for water shown in figure 2 and for other substances that contract during freezing (Note that water expands during cooling between 4oC and 0oC), shown in figure 3.
Figure2: p-v-T surface for water which expands on freezing
Figure 3: p-v-T surface of a substance which contracts on freezing
In both the figures, it will be observed that 3 equilibrium lines on p-T plane are regions bounded by saturation lines in the p-v and T-v planes. These saturation lines are saturated solid line, saturated liquid line and saturated vapour line. The saturation region between liquid and vapour contains a mixture of saturated vapour and saturated liquid. The quality x, of a vapour in the saturation region is the fraction of mass present in vapour phase. A mixture whose quality is less than 1 (or 100%) is called a wet mixture.
Conventionally ‘i’, ‘f’ and ‘g’ are used to designate the saturation properties in solid, liquid and vapour phases. Subscript ‘s’ is also used to designate the solid phase.
A vapour at a temperature higher than the saturation temperature corresponding to its pressure is called superheated vapour. A sub-cooled liquid is one which exists at a temperature lower than the saturating temperature to its pressure.
When we have made the above observations with reference to water, it would be noted that all pure substances exhibit the same general p-v-T behaviour.
When considering the behaviour of a substance in a given state, the state should be related to the critical point. If we are considering a substance in the region GH of figure 1, it is clear that liquid and vapour phase cannot exist in equilibrium because it is above the critical point.
A pure substance can exist in a number of different solid phases. A transformation from one solid phase to another is called allotropic transformation.
Phase Rule:
Gibbs’ phase rule states that the degree of freedom F of a mixture which is in equilibrium is given by
F=C – P + 2
Where C is the number of components in the mixture
P is the number of phases in which the equilibrium mixture exists
Therefore, a pure substance in a single phase (C = 1, P = 1) has 2 degree of freedom and therefore needs two properties to be specified to fix its state.
In the case of wet vapour (C = 1, P = 2), F = 1. That is only one property can be varied to maintain the same in the equilibrium region.
References:
Image sources
Figure 1: http://francescopochetti.com/wp-content/uploads/2014/01/phase.jpg
Figure 2: http://sounak4u.weebly.com/uploads/1/2/7/0/12702026/8795350_orig.png
Figure 3: http://www.crazymechanical.com/thermodynamic/properties-of-pure-substances/
In second part of this tutorial, we will explain Thermodynamics property tables, charts and Mollier Diagram,
One Response to “Properties of Pure Substances Part 1”
vipin
Hi, this is an exhaustive article on pure substances. i was looking for two property rule. and found it here